The dross currently represents a reaction product of the not completely mastered and controlled casting process. the studies and the researches on the issue allow however preventing the phenomenon, minimizing, or even completely eliminating, the effects.
Production processes can be affected by imperfections and therefore generate defects found in castings that can be both visible on the casting surface and made evident only after a machining or appear after fractures and/or dissections of the casting itself.
The main and most known defects in spheroidal graphite cast iron castings are: the graphite flotation, the pinholes, the presence of Chunky graphite or Dross inclusions, that’s to say non-metal inclusions: under conditions of bending and torsion fatigue, in which the cyclic stresses reach their maximum at the component surface, the fatigue strength is reduced by the presence of inclusions (as well as of slags, or of other defects or surface anomalies that in this case play the role of crack initiation sites).
Inclusions in castings: endogenous and exogenous
«The Dross, the slag and the sand», stated Adrian Udroiu, engineer working also as Satef consultant and already supporter and member of the Wedge Theory, «are the three non-metal inclusions that can be found in the majority of the situations in spheroidal graphite cast iron castings. The Dross ones represent, in particular, a manifestation of endogenous inclusion, where on the contrary the slag or the sand inclusions appear as inclusions of exogenous type».
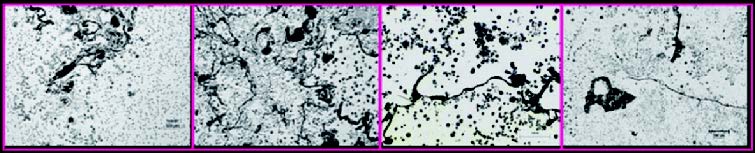
If analyzed under the microscope, Dross inclusions show a filamentous aspect. On the contrary, the slag and the sand appear in the form of blocks. However, the process through which such inclusions can be generated has not been understood, yet.
«The so called Dross», further added Adrian Udroiu, «are magnesium silicates that can be formed during the casting, in casting flow or also in the sprue basin, in the cast iron runners at the level of the first input cast iron. In their turn slags are instead generally generated by the magnesium treatment and they come directly from casting furnaces as residues».
The Dross constitutes therefore a reaction product formed by the magnesium treatment during the successive reoxidation phases of magnesium rejected by the cast metal in the moments that precede its solidification. It is therefore only a different term, useful for defining a specific slag type (that’s to say a reaction product). Going into details, the reaction binds magnesium with sulphur, oxygen and silicon, thus giving birth to continuous modules. This slag, in virtue of its low specific weight, is very light. And just for this reason it is generally present on the upper surface, or alternatively under the surface.
«It can also occur the event in which it is incorporated throughout the metal», specified Adrian Udroiu about this issue, «and this especially happens when colder casting temperatures occur».
It is however worth underlining that it is very difficult to avoid completely the magnesium reaction with the above mentioned, further, different elements (that’s to say sulphur, oxygen and silicon), since the presence of magnesium is absolutely necessary to form nodules.
An attentive observation of the slag under the microscope will reveal that it is very frequent to find also some graphite associated with it. This phenomenon intervenes owing to the insufficient quantity of magnesium, a quantity that differs depending on how much it will react with the other elements. It is also worth underlining that said slags appear under the form of long crosspieces instead of concentrates, like areas.
«In case the event occurred under the latter form», further specified Adrian Udroiu, «then slags behave the same way as cracks or graphite flakes in the structure. Such a conformation leads to a notable drop of the fatigue strength as well as to a decrease of the overall impact resistance of the material».
Theories about formation mechanisms
Dross generally shows a film appearance. Its formation is commonly and essentially associated with two events: the magnesium treatment and its reoxidation.
Concerning this, already in 1951, both the emeritus professor of Science of materials Richard Walter Heine and his colleague Carl R. Loper junior, both working at the University of the American Wisconsin State, gave an important contribution to the studies about the behaviour of liquid cast iron and to the explanation of the formation of complex films on the surface of the cast iron with spheroidal graphite cast iron.
After three years only, also the academics Merz and Marincek have contributed in the research bringing new information, and collaborating to the consolidation of the double film theory. On the basis of this theory it is in fact possible to explain what happens at the highest temperatures. When the liquid cast iron is at high temperatures, like for instance at 1,550°C, the Ellingham diaphragm indicates that the value of the carbon monoxide (or CO) is much more stable than the silicon dioxide (SiO2), and that therefore the carbon present in the bath oxidizes more rapidly than what instead happens to silicon.
«And it is just for this reason », further explained Adrian Udroiu, «that some gas bubbles can be easily observed on the cast iron surface in the casting furnace. At these temperatures, on the contrary, it is not possible to find silicon films. And the bath is clean. SiO2 particles are reduced to metal silicon, which will be dissolved into the metal. While in the meantime the CO is on the contrary expelled into the external environment».
At about 1,420°C the reaction balance is instead inverted, and the silicon oxidizes before carbon, forming a SiO2 film that covers the metal bath (the balance temperature depends on the chemical composition of the cast iron). At even lower temperatures and for instance at about 1,300°C, the Ellingham diaphragm highlights very clearly that for the cast irons containing manganese, the relative oxide (MnO) forms but it is characterized by lower stability than SiO2 and by even lower stability in comparison with carbon monoxide.
When preventing is better than correcting
On balance, the main elements that share in the Dross formation are then magnesium, (Mg), silicon (Si), iron (Fe) and oxygen (O2); complex chemical compositions that can give rise to silicon-free cast irons, without manganese and iron or finally in which oxygen is absent. We are talking about ingredients that are introduced in the various process phases in the operations of founding, spheroidizing, inoculation and casting.
«Just analyzing these phases and directly intervening on them», added the advisor of Satef, Mr Adrian Udroiu, «it is possible to prevent and to curb the Dross formation. Or, as an alternative, it is possible to operate in such a way as to reduce the concentration of inclusions».
Some remedies are for instance represented, in the order, by: de-oxidation and modification of the casting temperature of low-melting slags in the furnace with silicon carbide (SiC), reduction of the iron oxide (FeO) but not with barium (Ba); keeping the silicon percentage low (that’s to say within a threshold not exceeding 2%); or finally keeping magnesium under 0.40%. A further examined prevention method concerns the use of a moderate inoculation (ranging from 0.05 to 0.1%) with an exploitation of inoculants with elements with high affinity to oxygen, with low silicon content.
We should not forget, finally, the eventual suggestion of using closed risers for the contrast of the liquid shrinkage, or some ceramic filters close to the casting, besides using cast iron runners with wide section and therefore not presenting any turbulence.
Table: Chemical composition and some exemplifying micrographs of Dross inclusions
|
Analysis n° |
1 [%] |
2 [%] |
3 [%] |
4 [%] |
5 [%] |
6 [%] |
|
MgO |
25 |
14 |
16 |
48 |
40 |
24 |
|
SiO2 |
56 |
55 |
62 |
44 |
36 |
33 |
|
Al2O3 |
7 |
5 |
8 |
5 |
6 |
1 |
|
CaO |
0.5 |
– |
– |
– |
– |
– |
|
MnO |
5 |
23 |
11 |
– |
3 |
8 |
|
FeO |
4 |
3 |
2 |
2 |
16 |
31 |
Table: The table shows the effects of the fatigue strength on mechanical properties (Frauenhofer Institute/Refstie & Skaland– Vesta Group)
|
Defect |
Reduction of fatigue strength |
|
None |
1 |
|
Dross |
0.54 |
|
Micro shrinkage |
0.73 |
|
Macro shrinkage |
0.5 |
|
Chunky graphite |
0.75 |
|
Anomalies |
0.83 |



