In the oil and gas industry, scale has sometimes led to the complete shutdown, at least temporarily, of operating wells. So addressing this problem could have a big payoff.
Now a team of researchers at mit has come up with a potential solution to this huge but little-recognized problem. A new kind of surface treatment — involving nanoscale texturing of the surface, which is then coated with a lubricating liquid — can reduce the rate of scale formation at least tenfold, they have found. the findings are reported this week in a paper in the journal advanced materials interfaces written by graduate student srinivas subramanyam, postdoc gisele azimi, and kripa Varanasi, the doherty associate professor of ocean utilization in mit’s department of mechanical engineering.
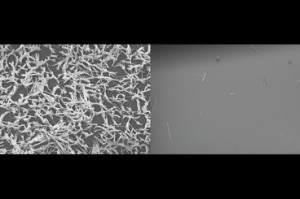
“You can see [scale] pretty much everywhere,” Varanasi says. In the home, these deposits are mostly an annoyance, but in industry they can cause “lost productivity, and the ways of removing [them] can be environmentally harmful,” typically involving the use of harsh chemicals. And in power plants and desalination plants, scale can cause significant efficiency losses, because it acts as a thermal barrier that impairs cooling or condensing in heat exchangers.
The problem arises because water often contains large amounts of dissolved salts and minerals. The ability of water to dissolve these materials depends on solubility, so if the water cools or evaporates, the solution may become supersaturated: it contains more of the dissolved material than it can accommodate, so some begins to precipitate out. It’s the same principle that causes fogging on a cold glass when warm, moist air cools suddenly as it meets a cooler surface.
For the most part, engineers have dealt with the problem by overdesigning systems, varanasi says: using pipes that are much larger than needed, for example, in anticipation of the partial blockages that scaling will cause, or a larger surface area, in the case of heat exchangers.
The problem is far from new, subramanyam points out: “Cooking pots from ancient times have this buildup” he says. “We don’t have good solutions yet.”
Though it remains to be proven on an industrial scale, the new approach developed by the mit team could make a significant difference in the rate of scale formation, and in many situations may prevent it altogether.
Their approach sounds deceptively simple: effective nanotexturing of the surface and filling the resulting textures with a lubricant. The texturing depends mostly on the scale of the bumps and grooves produced; the precise shapes don’t seem to matter. So a variety of techniques can be used to create that texture — including applying a textured coating to the surface, or chemically etching it in place.
The researchers also describe a process for selecting appropriate lubricants that can not only increase the energy barrier for scale formation, but also spread on the textured solid, making the surface “smooth” and reducing the nucleation sites available for scale formation.
Previous attempts to prevent or reduce scale formation have typically involved adding a coating (such as teflon) to a surface to prevent minerals from bonding to it. the problem with that approach, varanasi explains, is that these coatings can wear out, just as the coating on a nonstick frying pan often degrades with use. And if there’s even a pinprick of a hole in that coating, he says, that provides a place for scale to begin forming.
With the new method, once the nanotexture has been created on the surface, oil or another lubricating liquid is applied to that surface. the tiny nanoscale grooves capture this liquid, holding it firmly in place through capillary action, varanasi says. Unlike a solid nonstick coating, the liquid can flow to fill any gaps, spread on the surface textures, and can be replenished continually if some is washed away. “Even if there’s mechanical damage, the lubricant can return to that surface,” Subramanyam says. “It can maintain its smoothness for an extended period of time.” Because this lubricant layer is vanishingly thin — just a few hundred nanometers in thickness — protecting a surface for decades would require only a tiny amount of lubricant. Reservoirs built into a section of pipe could supply lubrication for the lifetime of the equipment, Varanasi says. In the case of oil pipelines, “the lubricant is already there,” and oil captured by the surface texturing could protect the pipe surfaces.
Jurgen Rühe, head of the laboratory for chemistry and physics of interfaces at the university of freiburg, who was not involved in this research, says this represents a “very significant finding and an important scientific advancement.” He calls the team’s approach to reducing scale formation “novel and creative” and says it “could have potential impact in all areas where water is heated and steam is generated.”
After further laboratory testing to determine the best lubricants and texturing methods for particular applications, this system could be ready for commercial applications in as little as three years, the researchers say.
The work was supported by the Mit Energy Initiative.



