 In order to store and use energy, platinum and other precious metals serve as catalysts to propel the most efficient fuel cells, but they are costly and rare. Now team of chemists from the University of Wisconsin-Madison introduces a new approach that uses a molecular catalyst system instead of solid catalysts. Although molecular catalysts have been explored before, earlier examples were much less efficient than the traditional platinum catalyst.
In order to store and use energy, platinum and other precious metals serve as catalysts to propel the most efficient fuel cells, but they are costly and rare. Now team of chemists from the University of Wisconsin-Madison introduces a new approach that uses a molecular catalyst system instead of solid catalysts. Although molecular catalysts have been explored before, earlier examples were much less efficient than the traditional platinum catalyst.
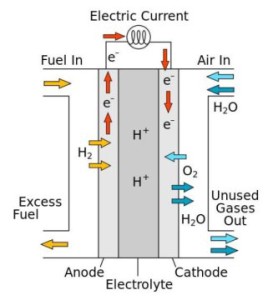
A fuel cell converts chemical energy into electricity by reacting hydrogen and oxygen at two different electrodes. A catalyst makes the reaction more efficient.
The new catalyst is composed of a mixture of molecules called nitroxyls and nitrogen oxides. These molecular partners play well together; one reacts well with the electrode while the other reacts efficiently with the oxygen.
“While this catalyst combination has been used previously in aerobic oxidations, we didn’t know if it would be a good fuel cell catalyst,” Professor Shannon Stahl says. “It turns out that it is the most effective molecular catalyst system ever reported.”
Because the approach involves chemical reactions between gases, liquids and solids, moving from concept to demonstration was no small feat. Gerken spent months studying and optimizing each component of the setup they had envisioned before testing everything in a model system.
“This work shows for the first time that molecular catalysts can approach the efficiency of platinum,” lab scientist James Gerken says. “And the advantage of molecules is that you can continue to modify their structure to climb further up the mountain to achieve even better efficiency.”



